About SELUTION SLR™
Breakthrough technology combining the proven safety and efficacy of sirolimus with advanced MicroReservoir and Cell Adherent Technology (CAT™) to deliver a sustained therapeutic effect;
- Controlled and sustained limus release for up to 90 days2
- Offering the broadest range of PTCA and PTA drug-eluting balloon catheters for coronary and peripheral indications.
Comparing a strategy of sirolimus-eluting balloon treatment to drug-eluting stent implantation in de novo coronary lesions in all-comers: Design and rationale of the SELUTION DeNovo Trial
The SELUTION DeNovo trial is a randomized trial comparing a PCI strategy with DEB and provisional DES to a strategy of PCI with systematic DES implantation. A total of 3326 patients will be included potentially making this the largest randomized trial performed with DEBs. The results of this strategy comparison with broad eligibility criteria will potentially have a major impact on PCI practice.
The SELUTION SLR™ drug-eluting balloon system for the treatment of symptomatic femoropopliteal lesions
An in depth review of SELUTION SLR 018 PTA Sirolimus Eluting Balloon Catheter in the treatment of symptomatic femoropopliteal lesions, including device design and technology and an evaluation of pre-clinical and clinical data.

SELUTION SLR 014 Sirolimus Eluting PTCA Balloon Catheter
Intended for use as a Percutaneous Transluminal Coronary Angioplasty (PTCA) balloon catheter to dilate de novo or restenotic coronary lesions, for the purpose of improving myocardial perfusion and decreasing the incidence of restenosis.
SELUTION SLR offers the broadest range of PTCA drug-eluting balloon catheters with balloon diameters ranging from 1.50 mm to 5.00 mm and lengths from 10 mm to 40 mm.
Learn More
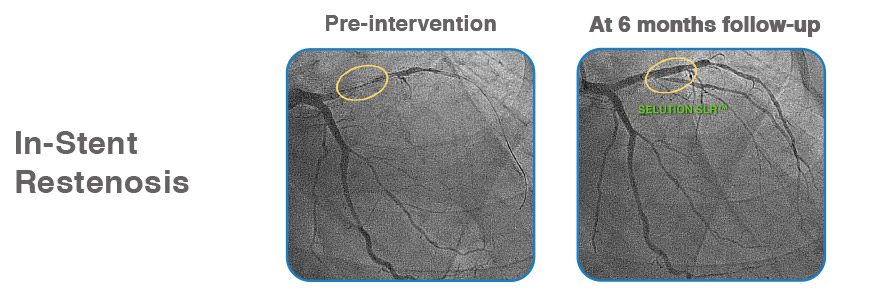
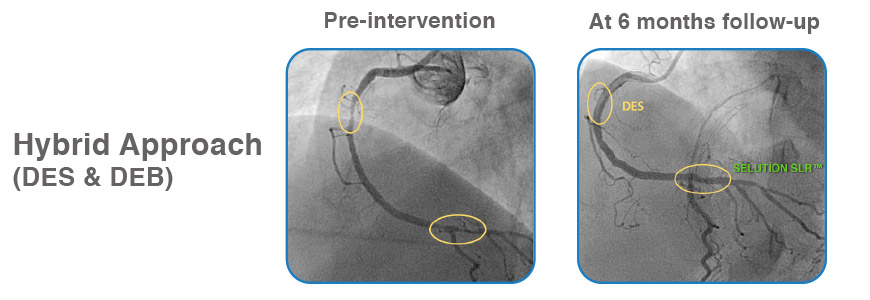
SELUTION SLR drug-eluting balloon (DEB) demonstrates safety and efficacy in First-in-Human coronary study3
-
Primary endpoint - 100% Freedom from device and procedure-related mortality through 30 days.
-
Low overall MACE rate of 2% at 12 months.
Case examples from First-in-Human Coronary Study
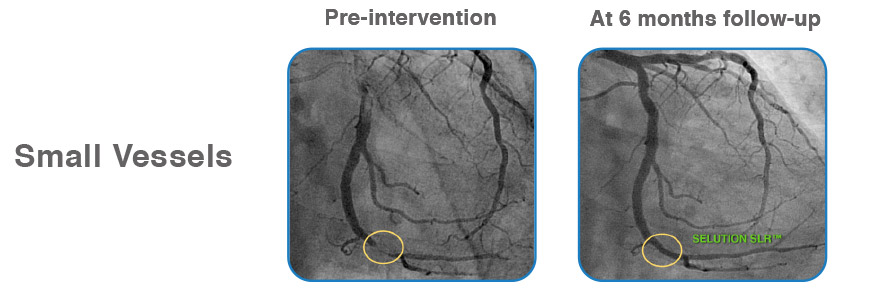
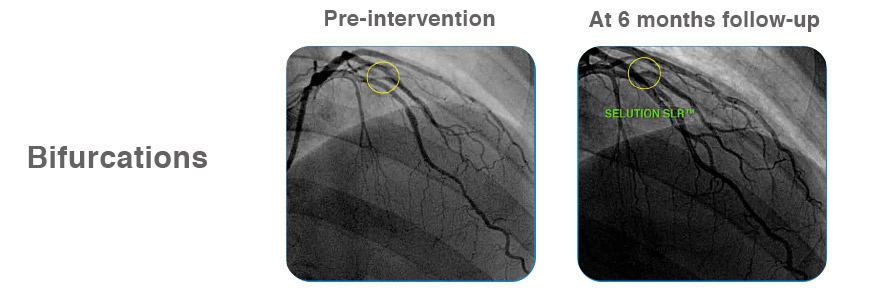
Learn more

SELUTION SLR 018 Sirolimus Eluting PTA Balloon Catheter
Intended for use as a Percutaneous Transluminal Angioplasty (PTA) balloon catheter to dilate de novo or restenotic vascular lesions, for the purpose of improving limb perfusion and decreasing the incidence of restenosis.
SELUTION SLR offers the broadest range of PTA drug-eluting balloon catheters with balloon diameters ranging from 2.00 mm to 7.00 mm and lengths from 20 mm to 150 mm.
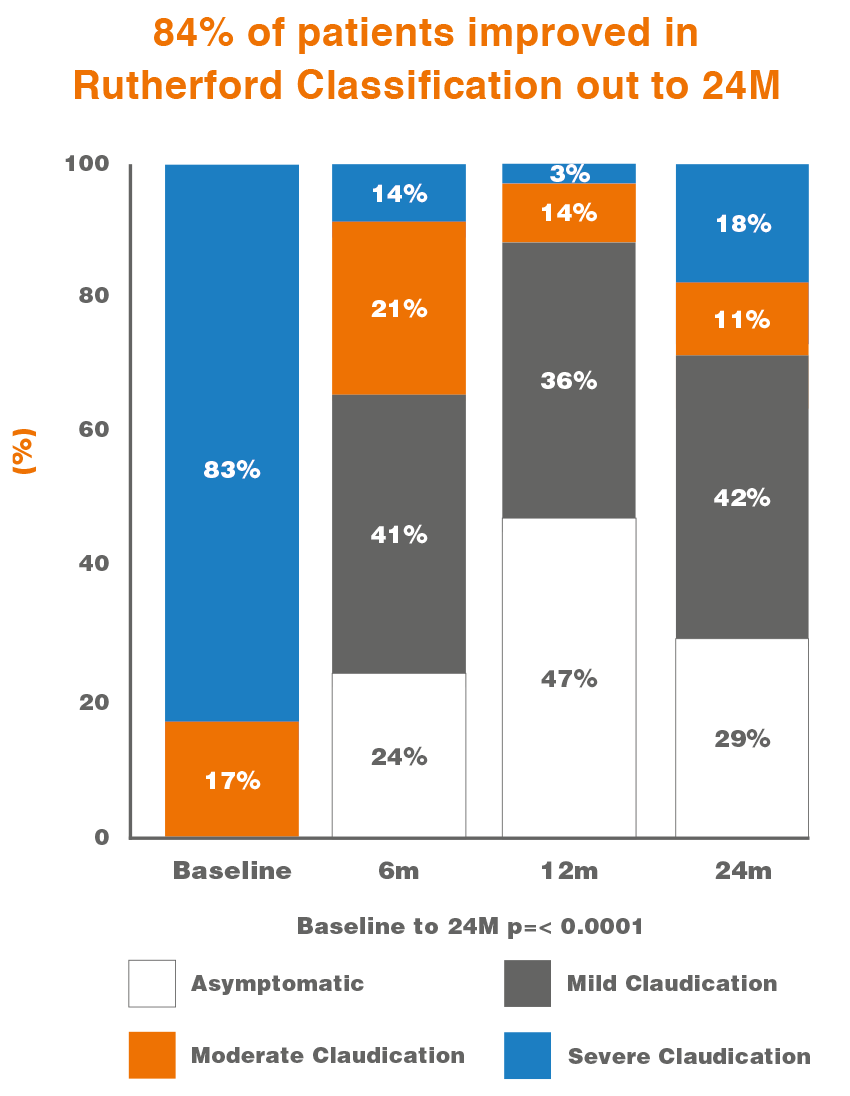
Delivering Long-Term Clinical Benefits in Peripheral Artery Disease 4
Principal Investigator: Thomas Zeller, MD, Germany (ClinicalTrials.gov ID: NCT02941224)
- Prospective, controlled, multi-center, open, single-arm clinical investigation
- 50 patients in 4 German Centers
- Met Primary Endpoint of Late Lumen Loss at 6 Months with 0.19mm**
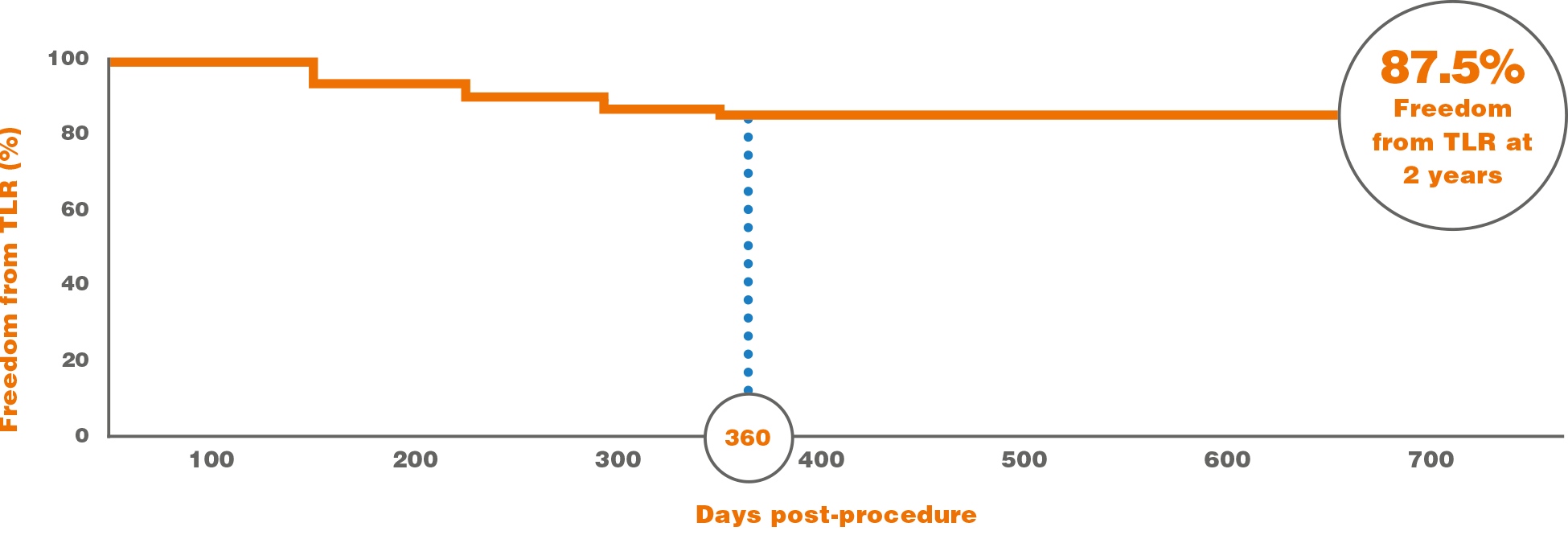
SELUTION SFA – Freedom from TLR

Data References:
- To achieve optimum performance from SELUTION SLR as a possible alternative to DES, follow the Instructions for Use in your implant procedure, and reference “How to use the drug-eluting balloon: recommendations by the German consensus group”, EuroIntervention 2011;7:K125-K128
- Drug concentration evident in MicroReservoirs and tissue – Data on file at M.A. Med Alliance SA.
- Windecker. S - Oral Presentation TCT 2019.
- Zeller, T; Oral Presentation, VIVA Conference 2019
** Presented as median value